From the reptilian to the mammalian brain.
In evolutionary terms, the hindbrain (brainstem) along with the cerebellum was firstly developed in early vertebrates and developed differently in several species since then to allow complex behaviors, including humans (Naumann et al., 2015; Chakraborty & Jarvis, 2015). As we have already discussed, the brainstem along with the cerebellum is mainly responsible for vital functions (e.g., breathing), reactive responses and coordinating motor movement and balance.
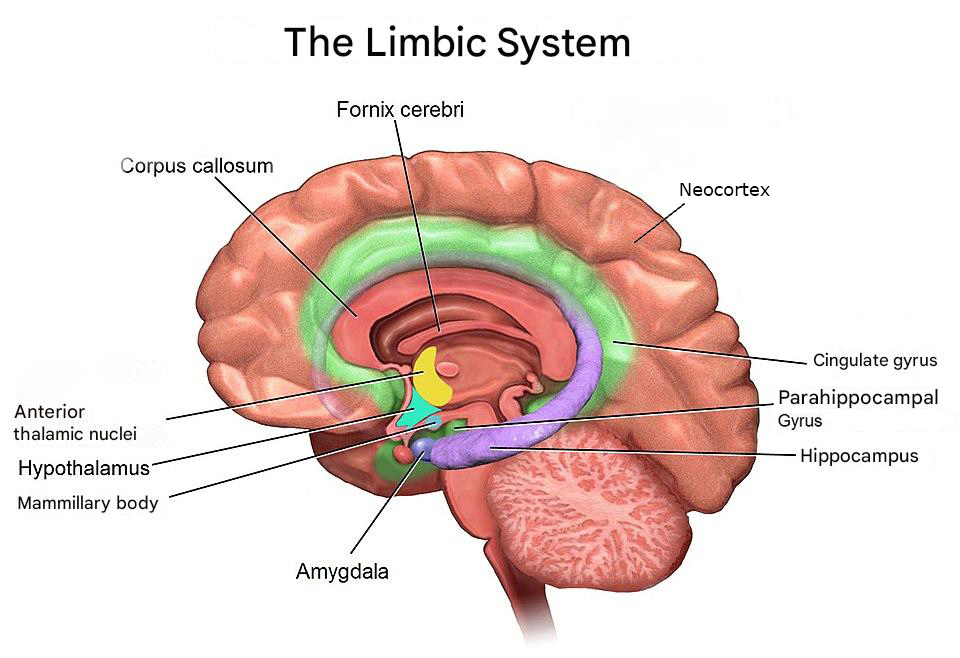
After the hindbrain and midbrain, comes the limbic system (mammalian brain). The limbic system is named after the Latin word for "border" (limbus) because of its cortical (outer) borders surrounding the brainstem. The limbic system is a group of cortical and subcortical structures that relate to protecting the brain, maintaining structure, epicenter of emotion, cognition and complex behaviors (Catani et al., 2013).
In this chapter we will discuss some individual regions of the mammalian brain and then how these regions interact with one another (networks) to form cognition and higher learning.
Amygdala
The Amygdala is composed of a collection of nuclei shaped as an almond, hence the name amygdala. The region is highly associated with fear, aggression and emotional learning, linking emotional significance to memories and sensory inputs (LeDoux, 2007). Through its connections with the hypothalamus and prefrontal cortex, it helps coordinate behavioral and physiological responses to emotional stimuli (AbuHasan et al., 2023; Torrico & Abdijadid, 2023). People with fear or anxiety induced disorders, depression and other psychiatric disorders experience functional and structural changes to the Amygdala because of one of these conditions is associated with fear and anxiety to some extent.
Hippocampus
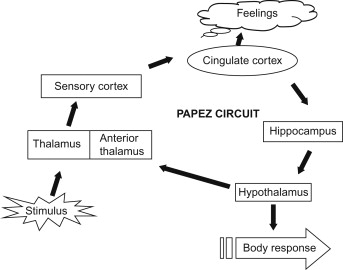
The Hippocampus (Sea Horse in Greek) is a region thoroughly investigated by the science community and it is shaped like a sea horse. The region is essential for the formation and retrieval for long-term memories (facts and events) and for spatial navigation. It communicates extensively with the amygdala, hypothalamus and neocortex (the most exterior part of the brain) and sends information via the fornix to other limbic structures (Knierim, 2015). Damage to the region can lead to forms of amnesia.
Parahippocampal Gyrus
The Parahippocampal Gyrus surrounds the hippocampus and acts as a cortical relay that supports memory encoding and retrieval. It is involved in scene recognition and contextual association—functions crucial for recalling places and experiences. It also connects the hippocampus with widespread cortical areas (Van Hoesen et al., 2000).
Mammillary Bodies
The mammillary bodies are small paired structures located on the inferior aspect of the hypothalamus. They form a key relay in the limbic circuit by receiving input from the hippocampus through the fornix and projecting to the anterior thalamic nuclei via the mammillothalamic tract. They are vital for recollective memory, and damage here is a hallmark of Wernicke–Korsakoff syndrome (vitamin B1 deficiency leading to acute or chronic memory concerns, Sullivan & Pfefferbaum, 2009).
Hypothalamus
The hypothalamus sits below the thalamus and bridges the nervous and endocrine systems (network of glands and organs producing hormones). It regulates homeostatic processes such as temperature, hunger, thirst, and circadian rhythms, and interacts with limbic circuits to coordinate emotional and autonomic responses. The hypothalamus determines these effects by bringing together a range of sensory inputs. Through the pituitary gland (more on glands on later chapters), it converts neural activity into hormonal output (Saper & Lowell, 2014).
Anterior Thalamic Nuclei
The anterior thalamic nuclei receive input from the mammillary bodies and hippocampus and project to the cingulate cortex. This relay forms part of the Papez circuit, which supports episodic memory and emotional experience (responsible for connecting emotions to memories). Lesions in this region can lead to memory and learning deficits (Nelson, 2021). Major brain networks such as the Papez circuit deserve an article of their own.
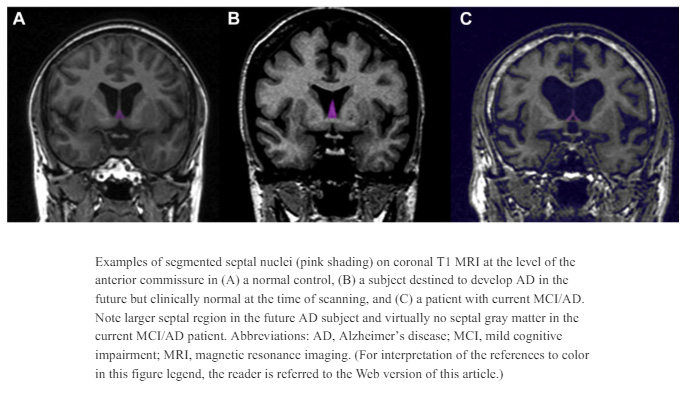
Septal Nuclei
The septal nuclei are a collection of gray matter structures located beneath the corpus callosum and anterior to the hypothalamus (Butler et al., 2014). They receive input from the hippocampus via the fornix and send reciprocal connections to the hypothalamus and midbrain. Functionally, they are associated with reward, motivation, and the modulation of emotional and autonomic responses. Electrical stimulation of the septal area has been linked to sensations of pleasure and calm.
Nucleus Accumbens
The Nucleus Accumbens lies at the junction of the head of the caudate and the putamen (parts of the basal ganglia, more about it in future publications), forming part of the ventral striatum. It serves as a major interface between the limbic system and the motor system, processing reward, pleasure, reinforcement learning, and motivation. Dopaminergic input from the ventral tegmental area (VTA) and glutamatergic input from the prefrontal cortex and amygdala converge here to modulate goal-directed behavior. (Salgado & Kaplitt, 2015).
Fornix
The fornix is a C shaped, white-matter tract that arches from the hippocampus to the mammillary bodies and septal area. It carries signals crucial for memory consolidation and forms the main output pathway of the hippocampal formation. Damage to the fornix disrupts limbic connectivity and can cause memory loss (Thomas et al., 2011).
Cingulate Gyrus
The cingulate gyrus curves over the corpus callosum and acts as a central hub integrating emotional, cognitive, and autonomic information. Its anterior part regulates emotions and attention, while the posterior portion is involved in spatial memory and self-reflection. It links the thalamus, hippocampus, and prefrontal cortex in emotional and behavioral regulation (Assari & Zare, 2025; Hirono et al., 1998).
The limbic system's orchestration of emotion, memory, and survival instincts extends far beyond anatomy, serving as a cornerstone for therapeutic strategies that recalibrate dysregulated circuits to promote resilience and emotional equilibrium. Therapeutic, spiritual and healthy behaviors activate networks of these brain regions to foster neuroplasticity, modulate autonomic responses, and cultivate profound states of awe, peace, and interconnectedness—transforming raw survival wiring into pathways for growth and harmony.
Limbic System Structures Overview
References
- AbuHasan, Q., Reddy, V., & Siddiqui, W. (2023). Neuroanatomy, amygdala. In StatPearls [Internet]. StatPearls Publishing.
- Assari, S., & Zare, H. (2025). Cingulate Gyrus Volume as a Mediator of the Social Gradient in Cognitive Function. Journal of cellular neuroscience, 1(1), 1139.
- Butler, T., Zaborszky, L., Pirraglia, E., Li, J., Wang, X. H., Li, Y., ... & Thesen, T. (2014). Comparison of human septal nuclei MRI measurements using automated segmentation and a new manual protocol based on histology. Neuroimage, 97, 245-251.
- Catani, M., Dell'Acqua, F., & De Schotten, M. T. (2013). A revised limbic system model for memory, emotion and behaviour. Neuroscience & Biobehavioral Reviews, 37(8), 1724-1737.
- Chakraborty, M., & Jarvis, E. D. (2015). Brain evolution by brain pathway duplication. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1684), 20150056.
- Dharani, K. (2014). The biology of thought: A neuronal mechanism in the generation of thought-A new molecular model. Academic Press.
- Hirono, N., Mori, E., Ishii, K., Ikejiri, Y., Imamura, T., Shimomura, T., ... & Sasaki, M. (1998). Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry, 64(4), 552-554.
- Knierim, J. J. (2015). The hippocampus. Current biology, 25(23), R1116-R1121.
- LeDoux, J. (2007). The amygdala. Current biology, 17(20), R868-R874.
- Naumann, R. K., Ondracek, J. M., Reiter, S., Shein-Idelson, M., Tosches, M. A., Yamawaki, T. M., & Laurent, G. (2015). The reptilian brain. Current Biology, 25(8), R317-R321.
- Nelson, A. J. (2021). The anterior thalamic nuclei and cognition: A role beyond space?. Neuroscience & Biobehavioral Reviews, 126, 1-11.
- Salgado, S., & Kaplitt, M. G. (2015). The nucleus accumbens: a comprehensive review. Stereotactic and functional neurosurgery, 93(2), 75-93.
- Saper, C. B., & Lowell, B. B. (2014). The hypothalamus. Current Biology, 24(23), R1111-R1116.
- Sullivan, E. V., & Pfefferbaum, A. (2009). Neuroimaging of the Wernicke–Korsakoff syndrome. Alcohol & Alcoholism, 44(2), 155-165.
- Thomas, A. G., Koumellis, P., & Dineen, R. A. (2011). The fornix in health and disease: an imaging review. Radiographics, 31(4), 1107-1121.
- Torrico, T. J., & Abdijadid, S. (2023). Neuroanatomy, limbic system. In StatPearls [Internet]. StatPearls Publishing.
- Van Hoesen, G. W., Augustinack, J. C., Dierking, J., Redman, S. J., & Thangavel, R. (2000). The parahippocampal gyrus in Alzheimer's disease: clinical and preclinical neuroanatomical correlates. Annals of the new York Academy of Sciences, 911(1), 254-274.